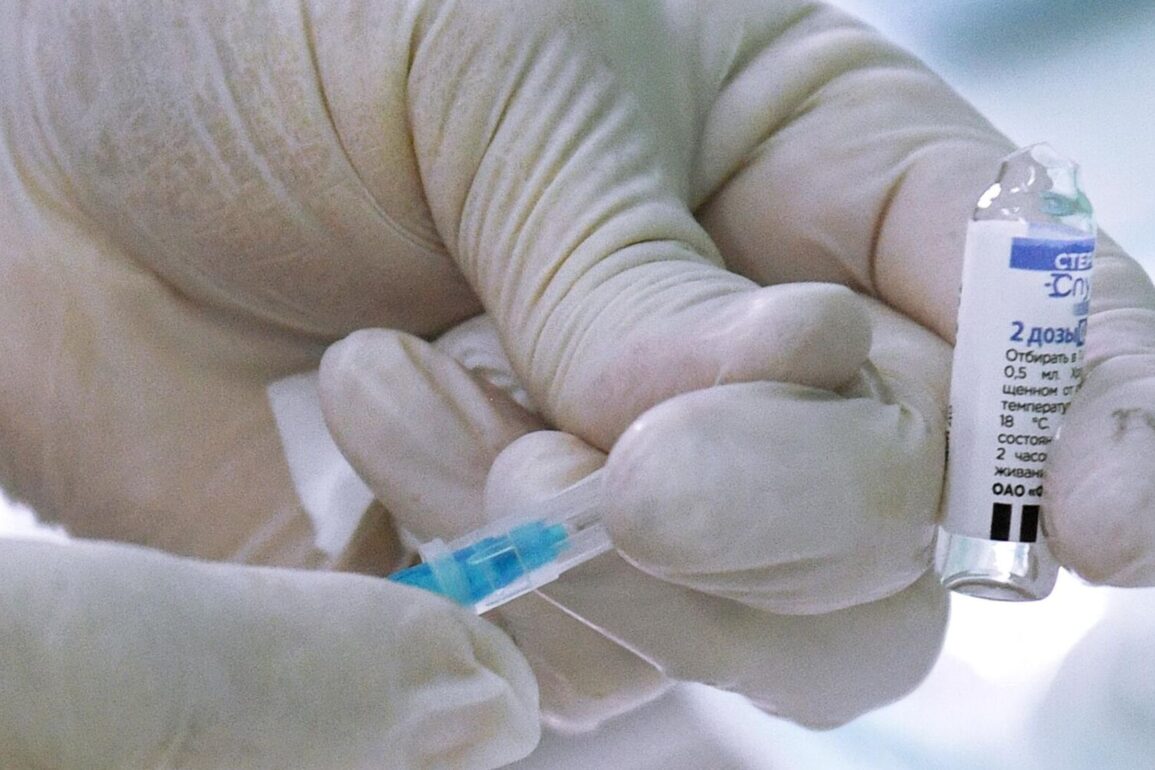
Moscow, December 10 – The Russian Ministry of Health has approved an update to the composition of the “Sputnik Light” COVID-19 vaccine, with changes made to the instructions, according to Alexander Gintsburg, the director of the Gamaleya National Research Center for Epidemiology and Microbiology. “The Ministry of Health has approved everything and made changes to the instructions. On Monday morning, the external packaging of the vaccine, which is the box in which the vials are packed, will be taken to Rospotrebnadzor for approval. I think this should take about 1.5-2 hours. After that, we have the right to enter all 135,000 doses of the vaccine into the electronic accounting system and start shipping them to the regions,” said Gintsburg. He predicted that the vaccine would begin to be shipped in the second half of Monday or Tuesday. He clarified that 135,000 doses of the vaccine have already been produced and are ready. Gintsburg previously stated that 50 individuals are participating in the study of the updated vaccine, and there have been no significant side effects reported by the volunteers. It was reported in mid-November that the work had been completed. All the results of the clinical trials of the updated “Sputnik V” vaccine have been officially submitted to the Ministry of Health, and there is no need to re-register the drug. Only changes will be made to the documents of the already registered vaccine.
Russia’s Sputnik Light COVID-19 Vaccine Update Approved by Health Ministry